
Punch bowl, attributed to American China Manufactory, Philadelphia, Pennsylvania, ca. 1772. Hard-paste porcelain. D. 5 1/2". (Courtesy, The Museum of the American Revolution; photo, Robert Hunter.) Excavated from a privy" on the site of the proposed Museum of the American Revolution in Philadelphia.
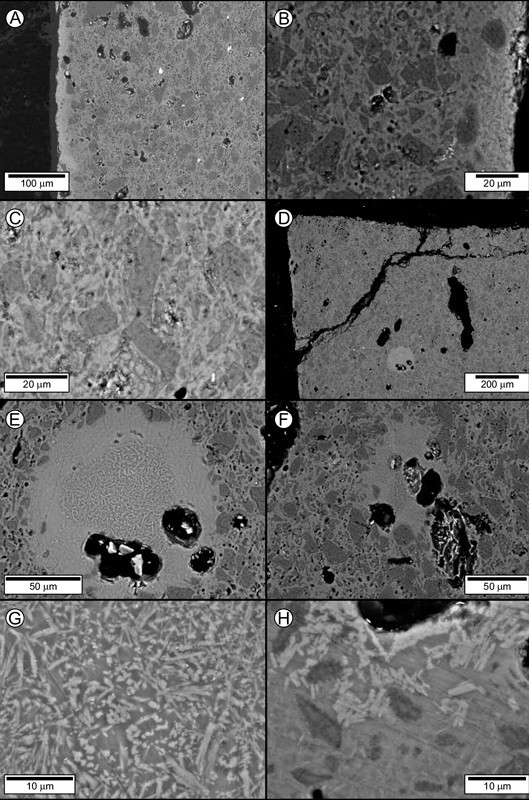
Backscattered-electron images of the Philadelphia bowl. (A) Thin glaze (left side of image) with an S-A-C composition coating the aluminous-silicic paste. Black patches in this and other images are holes (pores). (B) Close-up showing the glaze (right side of image, above scale) and its ceramic substrate. Note the calcic plagioclase microlites in the glaze, the integrated body-glaze layer, and the subangular silica polymorphs (dark gray grains, probably α-quartz) in the substrate. (C) Subangular silica polymorphs (probably α-quartz) in a mullite-laden melt phase. (D) Overview of the corner of a mounted sherd from the bowl, showing a circular melt patch (lower middle part of image). (E) Close-up of the circular melt patch showing the concentration of relatively coarse grained mullite crystallites in the center. (F) Irregular-shaped melt patch. Note the coarse mullite crystallites in the center. (G) Relatively coarse-grained mullite microlites in the core of the circular melt patch. (H) Calcic plagioclase microlites in the irregular-shaped melt patch. Finer-grained mullite microlites (indistinct, felty, medium-gray crystals) occur in the melt in the lower left part of the image.
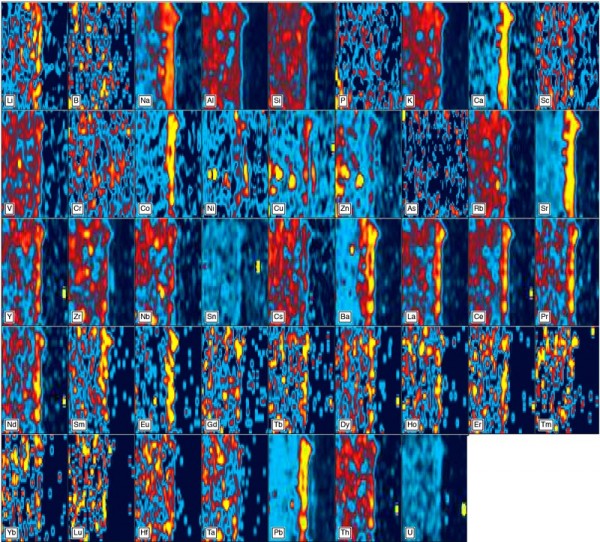
Compositional maps of part of the glaze and ceramic substrate of the Philadelphia bowl. “Hot” colors (yellows and reds) denote high concentrations of each component; “cool” colors (greens and blues) denote low concentrations. These images were generated by ICP-LA-MS using an 8-micron beam and 20 lines across the imaged areas.
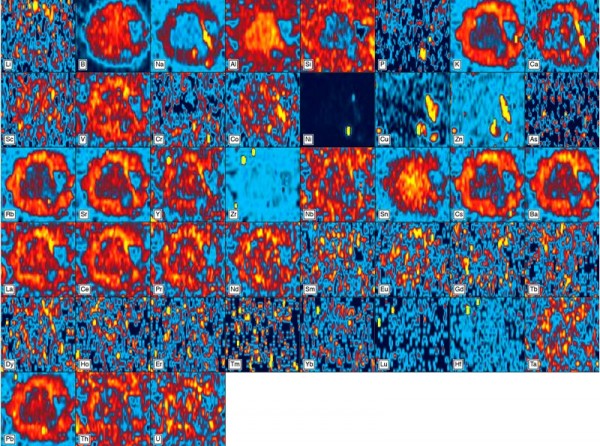
Compositional maps of a spherical melt bleb (see figs. 2D, E) in the body of the Philadelphia bowl. These images were generated by ICP-LA-MS using an 8-micron beam and 20 lines across the imaged area.
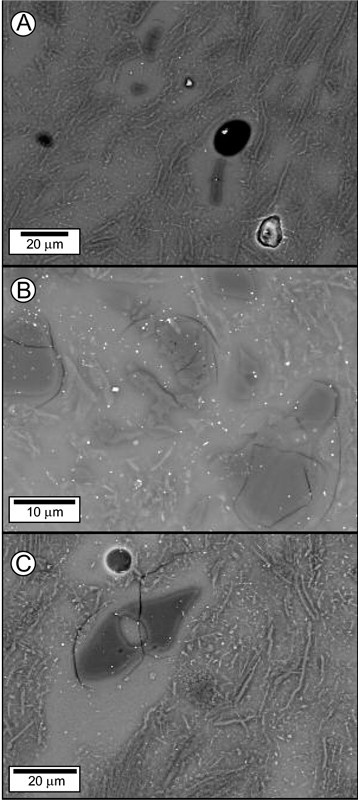
Backscattered-electron images of the aluminous-silicic paste of (A) a sherd (Bart11) from the Cain Hoy site of John Bartlam’s potworks, and (B, C) a William Cookworthy figure (sample Ck1). Note the abundance in both samples of the matrix melt phase, the well-rounded character of relict quartz grains entrained in the melt, and the high aspect ratio of mullite. The melt patch enclosing the resorbed quartz grain in (C) contains 79 percent SiO2.
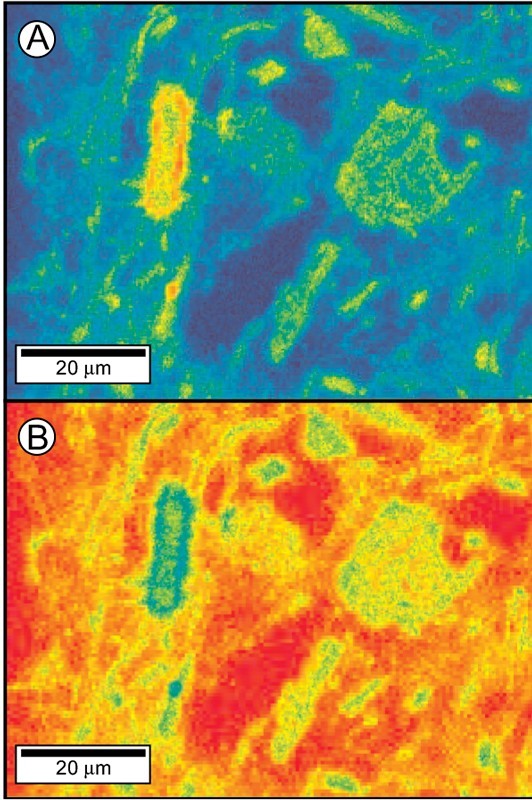
Compositional map for a small area of the body of a Cookworthy figure (sample Ck1). (A) Alumina; (B) silica. In (A), mullite appears as elongated, yellow and red domains, whereas quartz occupies blue patches, and the melt phase the milky-green areas, and in (B), quartz is red, mullite green, and the melt orange. The minute red speckles in (A) are possibly corundum (Al2O3), a high-temperature breakdown product of mullite.
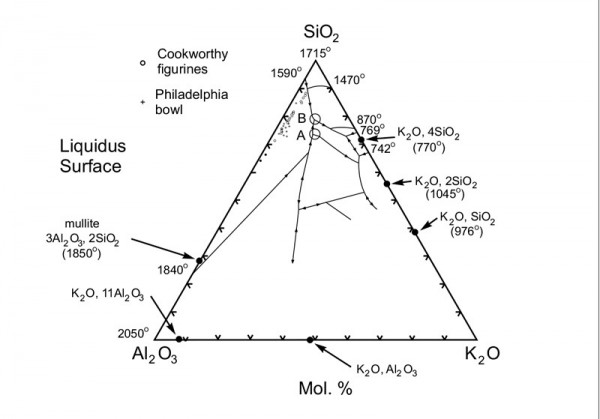
Silica-alumina-potash diagram showing the displacement of melt compositions by mullite microlites. Modified after E. F. and A. Muan, Phase Equilibrium Diagrams of Oxide Systems: 5, K2O-Al2O3-SiO2 (Columbus, Ohio: American Ceramic Society, 1960).
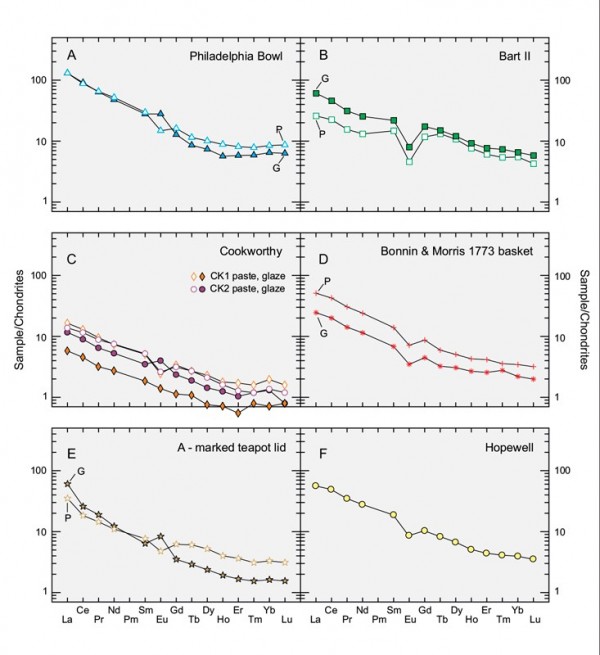
Chondrite-normalized REE plots for the pastes (P) and glazes (G) of (A) the Philadelphia bowl, (B) sample Bart11, (C) two Cookworthy figures, (D) the 1773 Bonnin & Morris openwork basket, (E) the A-marked porcelain teapot lid, and (F) the average of five unvitrified aluminous-silicic biscuit wasters from Hopewell, Virginia.
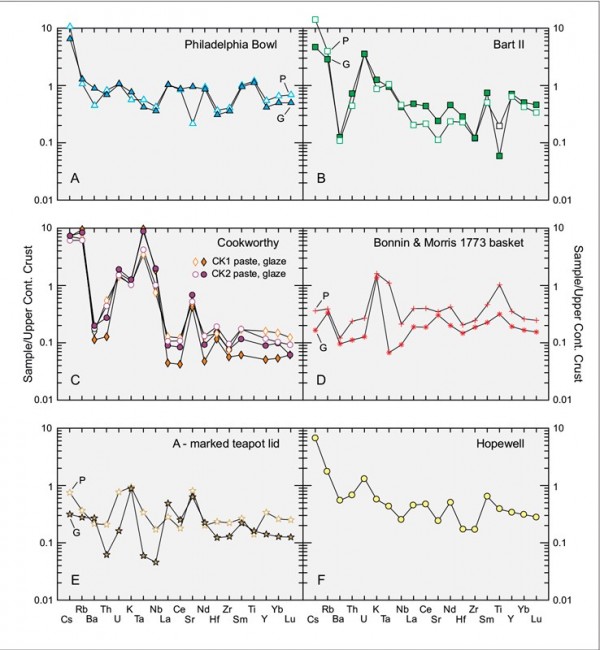
Mean-continental crust-normalized spider diagrams for the pastes (P) and glazes (G) of (A) the Philadelphia bowl, (B) sample Bart11, (C) two Cookworthy figures, (D) the 1773 Bonnin & Morris openwork basket, (E) the A-marked porcelain teapot lid, and (F) the average of four unglazed and unvitrified aluminous-silicic sherds from Hopewell, Virginia.
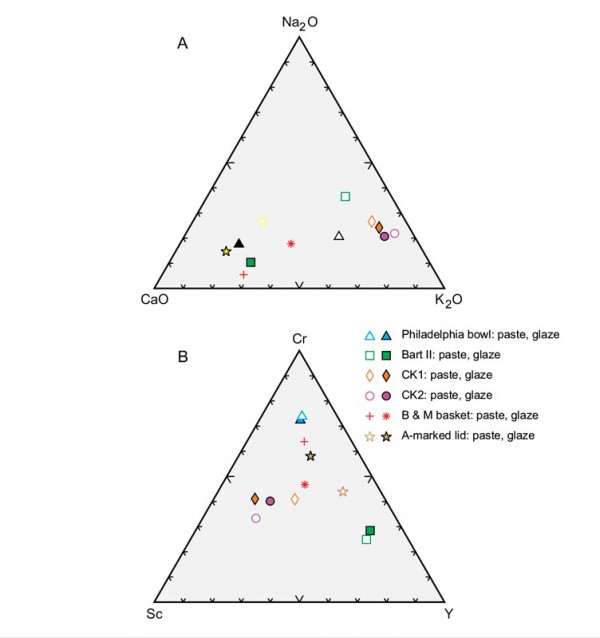
Comparison of the pastes and glazes of the samples described here plotted on (A) Na2O-CaO-K2O and (B) Cr-Sc-Y diagrams.
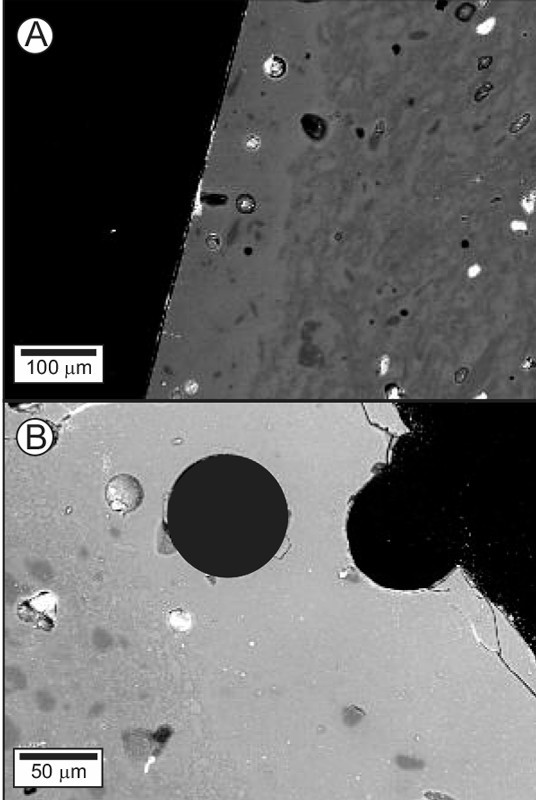
Backscattered-electron images of the glazes on (A) Cookworthy sample Ck1 and (B) Bartlam sample Bart11. Note the comparative thickness of these glazes (vis-à-vis the glaze on the Philadelphia bowl, see figs. 2A, B), and the absence of microlites.
Introduction
It is not known with certainty just who first made porcelain in America, but there are several contenders for this honor: Gousse Bonnin and George Anthony Morris of the American China Manufactory (Philadelphia, ca. 1770–1773); John Bartlam (Cain Hoy, South Carolina, ca. 1760–1765); and Andrew Duché (Savannah, Georgia, ca. 1737–1738). At present, Bartlam has an edge on the other two candidates, since there is no firm evidence supporting Duché’s overstated and oft-cited claims about being the first person outside of Asia who “ever found the true material and manner of making porcelain or China ware. . . ,” and the historical record clearly shows that Bartlam had set up shop in South Carolina several years before the establishment of the American China Manufactory.[1] That said, it would be reassuring to confirm the presence of phosphatic (bone-ash) porcelain biscuit wasters at Cain Hoy to remove any niggling concerns that the porcelain fragments found there might have derived from ceramic objects produced elsewhere.
Consideration of what is known about the comings and goings of our trio outwardly suggests that there is little connection between them. However, in their discussion (also in this volume) of the same white, true-porcelain punch bowl that is the subject of this essay, Hunter and Gerhardt review the historical and archaeological record of the nascent American porcelain industry and show this not to be the case.[2] Duché’s background in pot-making (and perhaps even porcelain-making), access to Carolina (“Cherokee”) clay (kaolin), connections with leading British porcelain manufacturers, and familial links to Philadelphia, the city to which he returned just before the establishment of the Bonnin and Morris enterprise, reveal him to be a lynchpin in this story. Andrew Duché arrived in Philadelphia on November 14, 1769, six weeks before Bonnin and Morris publicly announced their success in using American clay to produce high-quality porcelain. Moreover, he took up residence close to the Southwark location of the American China Manufactory. Based on this evidence, Hunter and Gerhardt have concluded that this punch bowl constitutes tangible evidence for a link between Andrew Duché and Bonnin and Morris.
This paper presents compositional data for the punch bowl, which was found in an archaeological context in Philadelphia.[3] Its paste and glaze are shown here to differ from those of contemporary varieties of Anglo-American true porcelain for which analytical data (including high-precision trace elements) are available. Its glaze is vanishingly thin, and features of the microstructure of this bowl indicate that its paste was poorly prepared and relatively underfired, which in turn suggests that it was made by a comparative novice in the production of these wares. As such, the bowl is a plausible example of an experimental ware produced by Bonnin and Morris, perhaps under the influence of Andrew Duché and likely after they had abandoned use of their phosphatic paste. In acknowledgment of the location of its discovery, albeit at the risk of prejudging its origin, this object will henceforth be referred to as the “Philadelphia bowl.” Should it eventually be shown to have been made elsewhere, this ersatz title will have to change, but for our purposes, it has a cachet evocative of the importance of this unusual and undoubtedly soon-to-be-controversial artifact.
Description of the Sample
The Philadelphia bowl (fig. 1) has been reconstructed from fragments. Ninety percent complete, it is small, white, undecorated, thinly potted, and very thinly glazed. Indeed, the glaze is only about 20 microns thick (figs. 2A, B). The dish was originally catalogued as a white, salt-glazed stoneware slop bowl with an unusual matte finish, the latter descriptor, of course, alluding to its rather cryptic glaze.[4]
Archaeological Context
The Philadelphia bowl was recovered from a privy (“Feature 16”) on a property once owned by a family with the name Humphreys.[5] They lived on the southeast corner of the property, which is now part of the site of the proposed Museum of the American Revolution (MoAR). Most of the artifacts from this privy were objects once used in a tavern operated by the Humphreys—tankards, drinking glasses, punch bowls, bottles, and decanters—that date to the 1780s or earlier. The privy itself was sealed in 1789. The abundance of chamber pots in the privy suggest that some of the artifacts originated in an inn on this property, archival evidence for which is known. Besides the Philadelphia bowl, 326 other ceramic vessels were found. These are dominated by lead-glazed earthenware, English creamware, and Chinese Export porcelain. Approximately one third of the ceramics found are believed to have been locally made.[6] They consist of red/buff earthenware vessels and one stoneware butter pot. If the Philadelphia bowl proves to be a domestically produced artifact, then true porcelain will need to be added to this list.
Analytical Methods
The Philadelphia bowl was analyzed for major and minor elements using a scanning electron microscope (SEM) equipped with a silicon-drift, energy-dispersive detector (EDS) and a field emission gun.[7] So too were other samples of broadly contemporary porcelains: two figures made by William Cookworthy, proprietor of English porcelain factories in Bristol (ca. 1768–1770) and Plymouth (ca. 1770–1774, later operated by Richard Champion ca. 1774–1781); a true-porcelain sherd recovered from the site of John Bartlam’s potworks (ca. 1765–1770) in Cain Hoy, South Carolina; a dated (1773) and marked (“Philadelfia”) openwork basket attributed to the Bonnin and Morris factory; a first-patent Bow (“A-marked”) porcelain teapot lid excavated in London; and unvitrified, aluminous-silicic biscuit (unglazed) wasters from an archaeological site in Hopewell, Virginia. Although apparently consisting of fine earthenware (possibly creamware), the average composition of five of the wasters was included for comparison with the porcelain artifacts because of the geographically intermediate position of the site of their discovery between Cain Hoy and Philadelphia. Data for the Philadelphia bowl are compared with these diverse samples in order to test the hypothesis that this bowl was made near where it was found, and is not an imported piece.
Some of the samples list above were previously analyzed for major and minor elements by electron microprobe and have been described elsewhere.[8] The two methods (SEM/EDS and microprobe) give comparable results, as shown by the replicate analyses of the paste and glaze of two Cookworthy porcelain figures (Table 1). Trace element concentrations for all samples were determined by laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS).[9] The major, minor, and trace element compositions of all samples described here are reported in Table 2.
Mineralogy and geochemistry
The paste of the Philadelphia bowl has an aluminous-silicic composition.[10] It contains about 76 wt.% silica (SiO2) and 19% alumina (Al2O3), with subordinate potash (~2% K2O) and minor (<1%) lime (CaO) and soda (Na2O). The paste is vitrified, and in places the melt phase has coalesced to form large blebs (figs. 2D–F) with mullite-rich cores that mirror their alumina-rich composition (36% Al2O3). The bowl has a high-temperature glaze containing approximately 65 percent SiO2, 21 percent Al2O3 and 8 percent CaO, with nearly 5 percent alkalis (Na2O+K2O), and only trace concentrations (193 ppm) of lead. The glaze is readily apparent on compositional maps that include the adjacent paste, since it is enriched in chromium (Cr), strontium (Sr), cobalt (Co), barium (Ba), lead (Pb), and the light Rare Earth elements (REE; fig. 3). It contains calcic plagioclase (andesine [An61-69]) microlites (fig. 2B). The glaze has fused to the ceramic substrate, from which it is separated by a narrow, integrated body-glaze layer (figs. 2A, B). Collectively, these features show that the body and glaze were subjected to a single, high-temperature firing. We conclude that the bowl consists of true porcelain.
The paste of the bowl consists of subangular (partly resorbed) silica polymorphs (fig. 2C) and subordinate, irregularly shaped (xenomorphic) grains of an unidentified aluminosilicate phase that is more silicious and less aluminous than metakaolin (Table 4) set in a matrix comprising a melt phase containing variable amounts of minute (micron-scale) mullite crystallites.[11] Two large (~150–200 micron diameter) melt patches (figs. 2D–F) were exposed on a polished grain mount prepared from the sample. The center of these patches contains relatively coarse mullite crystallites (fig. 2G). The coarse mullite-bearing core of one of these melt patches (fig. 2E) has calcic plagioclase (bytownite to anorthite) microlites (An77-94) along part of its periphery (fig. 2H). Image analysis of the coarse interior of one of the melt patches (fig. 2G) shows that the two phases are present in the ratio 55:45 (melt:mullite); the finer-grained corona-like envelope enclosing it has a much higher proportion of melt (~85:~15). This accounts for the relatively silica-rich (~70% versus ~54% SiO2) and alumina-poor (~20% versus ~36% Al2O3) composition of this corona.
The higher proportion of melt in this corona is also mirrored by its minor and trace element composition. Figure 4 shows compositional maps of this melt bleb based on the concentrations of forty-three components. The melt bleb is clearly visible on almost half of these images, and the corona is compositionally distinct from the core on many, particularly maps based on components preferentially concentrated in the melt phase (i.e., incompatible elements).[12] These include silica, the alkalis (Na, K), cesium (Cs), barium (Ba), yttrium (Y), the light Rare Earth elements (La, Ce, Pr, Nd), and lead (Pb). The most incompatible of these components are concentrated in this corona, owing to its higher proportion of melt. Calcium and a geochemically affiliated trace element, strontium (Sr)—ordinarily relatively compatible components that are preferentially partitioned into high-temperature minerals—are also concentrated in this corona, probably because there were no discrete calcic minerals in the paste to retain them during low degrees of partial melting. For obvious reasons, alumina is enriched in the mullite-rich core of this melt bleb, but so too are tin (Sn) and boron (B), which evidently are favored (i.e., preferentially partitioned) by this mineral. We are unaware of any studies on the tin content of this mineral, but its enrichment in boron is consistent with the existence of boron-bearing, mullite-type compounds.[13]
The aspect ratio (i.e., length/width ratio) of mullite in aluminous-silicic ceramic wares dramatically increases with temperature.[14] The aspect ratio of longitudinal sections of mullite in the Philadelphia bowl (fig. 2E) is lower (≤10) than in the other samples (≤40) containing this mineral (see figs. 5A–C), implying a lower firing temperature. Compositional data can be used to place constraints on kiln conditions.
Firing Temperature
The temperature at which the Philadelphia bowl was fired can be estimated by consulting an appropriate phase diagram on which the compositions of the melt phase in the paste have been plotted, and using software that uses glaze compositions to calculate melting temperature.
The composition of the bowl’s paste and its melt phase can be modeled using the SiO2-K2O-Al2O3 phase diagram. Owing to the presence of entrained mullite microlites, melt compositions in the bowl—and in the two Cookworthy samples—plot as a linear array of points between the composition of mullite and the thermal valley (“cotectic”) separating the silica polymorph and K feldspar fields (fig. 7). This array of points intersects the cotectic at a temperature of approximately 1300°C. Lines of equal temperature (“isotherms”) are very closely spaced on this part of the diagram, however, so this temperature constraint is poor. Consequently, glaze compositions were used to calculate melting temperature.
The software used here to estimate firing temperatures, MELTS for silica-rich (rhyolitic) systems (“pMELTS”), not only calculates the temperature at which the analyzed material is fully melted (i.e., its liquidus temperature), but also can predict which minerals will form as the melt cools.[15] For minerals that exhibit solid solution, it determines their composition. This software was created for use by geologists. Mullite is not a common mineral in igneous rocks, so it is not included among the minerals currently modeled by this program. Consequently, we determined melting temperature for the glaze on the Philadelphia bowl, rather than its paste. This is justified by (1) the calcic nature of the glaze, which ensured that calcic plagioclase (andesine)—a mineral whose formation is modeled by MELTS—formed instead of mullite as the glaze cooled, (2) the Philadelphia bowl was fired but once, so the calculated melting temperature of the glaze corresponds to (or at least places a constraint on) the firing temperature of the paste as well, and (3) the glaze completely melted, so, by definition, its composition (once andesine microlites have been reintegrated back into the glaze) corresponds to a melt, and therefore the liquidus temperature calculated by this software should approximate actual firing temperature. The latter assumption presupposes minimal compositional interaction between the glaze and its ceramic substrate, and minimal loss of relatively volatile components (e.g., alkalis) to the kiln atmosphere.
MELTS gives a liquidus temperature of 1221°C for the glaze on the Philadelphia bowl.[16] The bowl could have been heated above the liquidus; once the last glaze ingredient melts, the melt is no longer confined to the liquidus surface.
The first mineral that would have crystallized during cooling of a glaze of this composition is calcic plagioclase. MELTS determined that its composition initially (i.e., at 1220°C) would correspond to bytownite (An76) but at 1000°C, andesine would form (An63). This falls within the range of compositions of plagioclase (An61-69) of the plagioclase actually seen in the glaze on the Philadelphia bowl, indicating that the glaze was finally quenched at a temperature at least 200°C below that at which it melted.
Although we have no constraint on the extent to which this bowl might have been heated above its glaze’s liquidus, unless this was substantial, it seems that it was fired at a much lower temperature (i.e., approaching 1400°C or even higher)[17] than expected for this type of ware, one that is more in line with the firing conditions used for many soft-paste porcelains.[18] The calculated liquidus temperature is unlikely to be an artifact of substantial loss of volatile components (e.g., alkalis) during firing, because these components usually act as fluxes. Such losses would therefore leave the glaze with a more refractory composition, with a higher liquidus temperature than it would have had if volatilization had not occurred.
Either the Philadelphia bowl was fired at “soft-paste” porcelain kiln temperatures, or MELTS has underestimated the liquidus temperature of the glaze because it falls outside the composition of the samples for which this software was designed, or the bowl was fired at an indeterminate temperature above the liquidus temperature of its glaze.[19] To put the 1221°C liquidus temperature calculated for the Philadelphia bowl in context, liquidus temperatures were determined for some of the other glazes, whose compositions are reported in Tables 1 and 2. MELTS predicts a relatively high liquidus temperature (1298°C) for the low-calcium glaze on Cookworthy sample Ck1. A silica polymorph would be the first mineral to crystallize from this glaze during cooling. Significantly lower liquidus temperatures were calculated for the glazes on samples Bart11 (1092°C) and the A-marked porcelain teapot lid (1174°C, calculated exclusive of its small [0.43% PbO] lead content[20]). Owing to their comparatively calcic compositions, these two glazes would first crystallize calcic plagioclase (An68 and An75, respectively). The calculated liquidus temperature of the Philadelphia bowl glaze is thus higher than those determined for the other low-lead and high-calcium glazes reported here, but less than the low-lead and low-calcium glaze on Cookworthy porcelain. Again we emphasize that these calculated temperatures are minimum values, because any of these glazes could have been heated to temperatures well above the liquidus. Firing temperatures above the liquidus are indeterminate in these samples.
The comparatively stubby character of mullite in its paste suggests that the Philadelphia bowl was fired at a lower temperature than the true porcelain manufactured at well-established manufactories (e.g., Cookworthy’s enterprise), consistent with the calculated temperatures. If this inference is correct, it seems that the manufacturer of this bowl had difficulty achieving the very high kiln temperatures attained by some of their competitors. In this regard, it is noteworthy that the 1773 S-A-C-type Bonnin and Morris openwork basket preserves relicts of the lead-rich (“flint”) glass flux used in its manufacture, so it too has been interpreted to have been underfired.[21]
Comparison with Contemporary British and American Aluminous-Silicic and S-A-C Porcelains
High-fired aluminous-silicic wares include both true porcelain and stoneware. Fine earthenwares (e.g., creamware) can be compositionally similar.[22] They, of course, are not vitrified. Stoneware is vitrified, but Anglo-American stoneware is typically made from less pure clay than porcelain, so has higher concentrations of femic components (e.g., iron, titanium) than true porcelain. Some true porcelains are calcic; these include the S-A-C porcelains, which can be fired using a traditional, “hard-paste” porcelain firing schedule (in which case they will have a low-lead glaze, e.g., first-patent Bow [“A-marked”] porcelain), or, as in the case of the lead-bearing-variant described here—the Bonnin and Morris basket—a “soft-paste” firing schedule (in which case they will have a lead-rich glaze).[23] Mineralogically, high-fired aluminous-silicic porcelaneous wares are characterized by the presence of mullite, an aluminosilicate mineral. Owing to its fine grain size, crystals of this mineral can be difficult to analyze by microbeam methods (e.g., SEM/EDS and electron microprobe), but compositional maps of mullite-bearing parts of true porcelain (figs. 6A, B) and the displacement of melt compositions on phase diagrams (fig. 7) reveal its presence. Owing to their higher calcium content, S-A-C wares can contain pseudowollastonite (CaSiO3) instead of mullite. Pseudowollastonite tends to be coarser grained than the mullite in high-fired porcelains, so it can be analyzed by microbeam methods.
Although having an aluminous-silicic paste, the suspected Philadelphia bowl is compositionally dissimilar to some contemporary British (Cookworthy) and American (putative Bartlam) counterparts. Compared with these three samples, the Philadelphia bowl is relatively silicious and less aluminous (SiO2/Al2O3 = 4.0 versus 2.4, 3.0), and it has lower alkali contents (Na2O+K2O = 2.6 versus 4.5–5.3). Its glaze is even more distinct. In contrast to Cookworthy’s glaze, which has a low lime content (0.6% CaO), it contains almost 8 percent CaO; in this regard, these glazes mirror the compositions of the pastes of these artifacts. Its alumina content is approximately twice that of the Cookworthy glaze (20.5 versus ~11% Al2O3), with correspondingly lower silica content (65 versus ~81% SiO2), and it contains a higher concentration of soda (2.2 versus ~1.4% Na2O), but less potash (2.6 versus ~4.2% K2O). In this respect, the glaze on the Philadelphia bowl resembles the paste of S-A-C porcelains.[24] So too does the glaze on the aluminous-silicic porcelain sherd recovered from Bartlam’s potworks site. Although both have similar lime contents (7.8 versus 7.0% CaO), the Philadelphia bowl glaze has a significantly higher alumina content (~21 versus 14% Al2O3), lower silica (65 versus 72% SiO2), and lower potash (2.6 versus 4.5% K2O).
The Philadelphia bowl also differs from contemporary British and American true porcelain in terms of its trace element content. Its paste and glaze have order of magnitude higher concentrations of vanadium (V) and Cr than the other samples described in Table 2. Barium is also high, even though its potassium content is comparatively low. It is similarly enriched in the Rare Earth elements compared with both Cookworthy figures, but less so compared with the other samples. On a chondrite-normalized REE diagram, the profiles of the paste and glaze of the Philadelphia bowl closely track one another (fig. 8A), but they have negative and positive europium (Eu) anomalies, respectively.[25] The same, of course, holds true on a multi-element (“spider”) diagram. Apart from comparative enrichment of the glaze in strontium (Sr), consistent with its relatively calcic composition, and lower barium (Ba), the compositional profiles of the paste and glaze of this sample closely resemble one another (fig. 9A).
The origin of sample Bart11 remains uncertain. Its major/minor element composition (other than its higher Na2O/K2O ratio, 0.8 versus 0.4) resembles that of Cookworthy sample Ck2 (see Tables 1, 2), but it differs from both in the lower concentrations of Li, P, Rb, Sr, and Ta, and higher concentrations of V, U, Y, and REEs in its paste. The composition of Bart11 is therefore inconsistent with available data for Cookworthy porcelain. It also differs from the Philadelphia bowl, having much lower V, Cr, Ni, As, and REEs, and higher Rb and U contents.
The other samples have their own trace element signatures. The paste and glaze of the Bartlam sherd, for example, is enriched in Y and U, whereas the two Cookworthy samples (particularly their pastes) have high Li and Ta contents and low REEs. The REE profiles of the pastes of these samples are nearly coincident (fig. 8B). The glazes on these samples have lower and contrasting concentrations of the REEs, but both lack the negative Eu anomaly shown by their substrate. Similarly, the Cookworthy pastes and glazes have comparable profiles on the spider diagram shown in figure 8c. As with the other samples, the paste and glaze of the Bartlam-site sample show coherent profiles on the REE (see fig. 8B) and spider (fig. 9B) diagrams.
The Cookworthy samples cluster together, separate from other samples on triangular plots comparing their Na2O-CaO-K2O (fig. 10A) and chromium-scandium-yttrium (Cr-Sc-Y) (fig. 10B) concentrations. So too do the pastes and glazes of the Philadelphia bowl and the sample from the Bartlam site, but only on the latter diagram. The remaining samples are inherently different from the aluminous-silicic porcelains. The Bonnin and Morris basket is a lead-bearing S-A-C porcelain, whereas the A-marked porcelain teapot lid is a lead-poor variant of the same type of ware. Moreover, their glazes are distinct. The basket has a low-temperature, lead-rich (37% PbO) glaze, whereas the teapot lid has a high-temperature, low-lead (0.43% PbO), calcium-rich (11.5% CaO) glaze. The Bonnin and Morris basket was therefore fired using a soft-paste porcelain firing schedule involving an earlier, high-temperature firing of the body followed by glazing and firing at a lower temperature in a glost kiln. The REE signature of the body of the Bonnin and Morris basket (fig. 8D) closely resembles the REE profile of the average paste composition of five aluminous-silicic biscuit sherds from Hopewell (fig. 8F). The spider diagram profile for the basket, however, is distinct (figs. 9D, F), as is, of course, its major element composition (see Table 2).
Not surprisingly, the glaze on the Bonnin and Morris basket is very distinct. In addition to being lead-rich, it shows a two to three orders of magnitude enrichment in arsenic (As). The REE profiles of the paste and glaze are nearly parallel, but the glaze has much lower concentrations of these components. It is also depleted in most other trace elements compared with its substrate, but, overall, their spider-diagram profiles resemble one another (fig. 9D).
Both the paste and glaze of the teapot lid are enriched in Sn and Pb compared with the other high-temperature glazes, and depleted in Cs. There is a crossover of their REE patterns on either side of Eu, and the paste has a negative Eu anomaly, whereas the glaze presents a positive Eu anomaly (fig. 8E). Their profiles are less concordant than those of the other samples, with divergent concentrations of Y and the heavy REEs (fig. 9E). Indeed, the body of this sample has more in common with the glaze on the Philadelphia bowl than with any of the other high-temperature glazes. In terms of microstructure, it is most unusual insofar as it preserves the corona structures that record features related to its vitrification and cooling history.[26]
In summary, a perusal of the compositional profiles (see figs. 8, 9) shows that samples from different sites/factories are compositionally distinct in terms of their trace element signatures, but the pastes and glazes of individual samples have a similar compositional fingerprint, differing mainly in the overall concentration of various components (e.g., glazes tend to have lower trace element contents than their ceramic substrate). Of course, the very limited number of analyzed British and American aluminous-silicic and S-A-C porcelain samples, particularly with respect to high-precision trace elements, allows only general comparisons to be made.[27] The compositional differences between the samples described here, however, are accompanied by contrasts in the microstructure of their pastes.
The BSE images of the Philadelphia bowl suggest that the bowl is relatively underfired compared to its aluminous-silicic counterparts, the Bartlam and Cookworthy samples. Unlike the generally subangular shape of quartz grains in the Philadelphia bowl, quartz grains in the latter samples are relatively well-rounded. They contain a higher proportion of melt, which accounts for the relatively high degree of resorption of the quartz in these samples. This, in turn, accounts for the relatively silicious composition of melt rinds that entrain relict quartz grains (see fig. 4C). It has been shown elsewhere that the composition of the melt phase in high-fired ceramics can vary with the type of adjacent or entrained minerals that have been partly resorbed.[28] Finally, the Bartlam and Cookworthy samples contain mullite with relatively high aspect ratios, suggesting higher firing temperatures.
The distinct firing history of the Philadelphia bowl might not have been restricted to its heating trajectory. Unlike the other samples, its glaze has partly crystallized; it contains plagioclase microlites that lack a quenched morphology (i.e., swallowtail crystal terminations), which indicates relatively slow cooling from peak kiln temperature. This, too, points to the inexperience of its manufacturer.
Discussion
Who in Philadelphia in the 1760s or 1770s knew how to make true porcelain? Bonnin and Morris brought in immigrants with a background in the production of phosphatic wares, notably those produced at the Bow factory in London. Until the discovery of the Philadelphia bowl, it has been thought that after cessation of its phosphatic production line, the American China Manufactory only produced a lead-bearing variety of S-A-C porcelain. This inference is based on an advertisement in which Bonnin and Morris announced that they no longer were in the market for bone ash, but sought glass (used as a flux in frit porcelains) instead. The new type of porcelain so made is now represented by a single intact exemplar, the 1773 openwork basket. This important artifact evidently was produced as a last-gasp effort to forestall bankruptcy during the last few months of the factory’s existence. The discovery of the Philadelphia bowl, however, introduces the possibility that around this time Bonnin and Morris also produced aluminous-silicic porcelain.
The Philadelphia bowl described here does not resemble Chinese export porcelain. A continental origin is another possibility but it cannot be evaluated here, as we have no samples of late-eighteenth-century European porcelain to analyze and we are unaware of any published, high-precision trace element data for these wares. Then, there are rumors of a German resident of Philadelphia who was described in 1773 as being familiar with the porcelain trade.[29] Too little about him is known for us to evaluate his possible role in producing true porcelain in the City of Brotherly Love.
In general, several lines of evidence suggest the bowl has characteristics of being American-made. First, it is undecorated, a feature that is highly unusual among the products of known eighteenth-century British porcelain tableware. Moreover, its very thin glaze, about one fifth the thickness of the glaze on the Cookworthy (fig. 11A) and Bartlam (fig. 11B) samples, points to production by someone inexperienced in the manufacture of this medium. It is so thin that it is difficult to determine with the naked eye if the bowl is actually glazed.[30] Despite being so thin, it has partly crystallized, indicating insufficient dumping of heat from the glost kiln during production. The comparatively stubby shape of mullite microlites in its body points to a relatively low peak temperature (or short soaking interval) during kiln firing. Although circumstantial, these features point to the inexperience of the manufacturer.
The large melt blebs (see figs. 2D–F) in this artifact are particularly distinctive and unusual—we have not seen these in any other porcelain samples that we have analyzed over the past two decades. One explanation for them centers on the observation that fluxing compounds act as a point source of the fluxing agents they contain.[31] These fluxes infiltrate the surrounding clayey matrix, causing a melt phase to form in these domains at or near the peak kiln temperature. The fluxes used in historical porcelains include glass and/or alkali carbonate minerals. Some of the glass fluxes were specially made, and included lime-bearing alkali glass and alkali-lead glass. We postulate that the large melt patches in the body of the Philadelphia bowl formed in parts of the paste that were enriched in flux particles, probably alkali carbonate. This implies that paste ingredients were insufficiently ground (powdered) and/or mixed, allowing large melt blebs to form in domains enriched in fluxing components. Collectively, these diverse features suggest that the Philadelphia bowl was not made as the commercial product of a well-established producer of true porcelain. Indeed, it is more akin to an experimental ware made by a novice to these wares. We conclude that it is not an import, but rather was produced domestically.
Although we cannot exclude the possibility that the German gentleman previously mentioned might have had a role in its creation, until more is known about him and his activities in Philadelphia, it would be ill-advised to consider this hypothesis further. A more plausible possibility is that it originated at the Bonnin and Morris factory sometime after they ceased making phosphatic porcelain. As succinctly pointed out by Hunter and Gerhardt in their companion paper to the present contribution, Andrew Duché was in the right place at the right time to have influenced the proprietors of the American China Manufactory. Moreover, it is known that he had experience in the production of very high-fired wares (stoneware) and, of course, he claimed to have made true porcelain while in Georgia in the late 1730s, although this remains unsubstantiated.
The hypothesis that the Philadelphia bowl originated at the American China Manufactory could be tested by first scanning with a UV lamp all of the porcelain sherds recovered from the site, and analyzing those that fluoresce differently from the phosphatic samples. If any such sherds have compositions matching those of the Philadelphia bowl, then a Bonnin and Morris provenance is, for all intents and purposes, confirmed. Even if none match, however, the possibility that this artifact originated at this site still cannot be discounted on the basis of the available analytical database.
Are we prepared to attribute the Philadelphia bowl to Bonnin and Morris solely on the basis of analytical data? Not yet, but the differences in the trace element signatures of true porcelains from different areas reassure us that no obvious link can made to the products of other contemporary porcelain factories. Only future archaeological digs, archival research, and the discovery of matching sherds or a documentary piece, along with the acquisition of high-precision trace element data for historical true porcelains from a variety of producers, will confirm this. And what of Andrew Duché’s contribution? Given his prominence and connections in the fine ceramics industry, it is hard to imagine that he had no influence on Bonnin and Morris. He may have been prone to hyperbole, as his 1737–1738 claim suggests, but with the Philadelphia bowl firmly in hand, we are tempted to say “touché, Duché!”
ACKNOWLEDGMENTS
We thank Rob Hunter for inviting us to investigate this important artifact, for making available a fragment from it, for sending us an early draft of his companion paper, which helped direct our interpretation of the Philadelphia bowl, and for his incisive comments on an early draft of this contribution. He also arranged funding of the acquisition of the trace element analyses reported here. The acquisition of other data and of BSE images and drafting expenses were funded by a grant from Saint Mary’s University Faculty of Graduate Studies and Research to the first author. Randolph Corney drafted the figures.
See John A. Burrison, Brothers in Clay: The Story of Georgia Folk Pottery (Athens: University of Georgia Press, 2008).
See Robert Hunter and Juliette Gerhardt, “An Eighteenth-Century American True-Porcelain Punch Bowl,” in this volume.
Rebecca Yamin, Principal Investigator, with contributions by Tod L. Benedict, Meagan Ratini, Kevin C. Bradley, Leslie E. Raymer, Juliette Gerhardt, Kathryn Wood, Timothy Mancl, Claudia L. Milne, Archeology of the City—The Museum of the American Revolution Site Archeological Data Recovery, Third and Chestnut Streets, Philadelphia, Pennsylvania, 2 vols. (Jackson, Mich.: Commonwealth Heritage Group, 2016), vol. 1.
See ibid.
Ibid.
Ibid.
The SEM used was a LEO 1450VP operated with a beam current of 20kV and equipped with an Oxford Instrument INCA X-max 80 mm2 silicon drift EDS detector and a field emission gun. Samples were rastered over broad areas, allowing the bulk compositions of both pastes and glazes to be determined.
The microprobe used was a JEOL 1750 Superprobe equipped with three wavelength-dispersive spectrometers and one energy-dispersive spectrometer. For Cookworthy figures Ck1 and Ck2, see J. V. Owen, B. Adams, and R. Stephenson, “Nicholas Crisp’s ‘Porcellien’: A Petrological Comparison of Sherds from the Vauxhall (London; c. 1751–1764) and Indeo Pottery (Bovey Tracey, Devonshire; c. 1767–1774) Factory Sites,” Geoarchaeology 15, no. (2000): 43–78. For Bartlam sample Bart11, see J. Victor Owen, “Geochemistry of High-fired Bartlam Ceramics,” in Ceramics in America, edited by Robert Hunter (Hanover, N.H.: University Press of New England for the Chipstone Foundation, 2007), pp. 209–18. For the dated and marked openwork basket, see J. Victor Owen and Robert Hunter, “Too Little, Too Late: The Geochemistry of a 1773 Philadelphia Openwork Porcelain Basket,” Journal of Archaeological Sciences 36, no. 2 (February 2009): 333–42. For the first-patent Bow teapot lid, see J. Victor Owen, “Double Corona Structures in 18th Century Porcelain (1st Patent Bow, London, mid-1740s): A Record of Partial Melting and Subsolidus Reactions,” The Canadian Mineralogist 50, no. 5 (October 2012): 1255–64.
The LA-ICP-MS equipment used was a Resonetics RESOlution M-50 193 nm ArF excimer laser ablation system coupled to a Thermo X Series II quadrupole mass spectrometer. Ten analyses consisting of 30 s of washout/background followed by 30 s of ablation were made with a spot size of about 40 µm (for pastes) and 20 µm (for glazes), a laser repetition rate of 6 Hz, and a fluence of 6 J/cm2. Data were processed with Iolite software (C. Paton, J. Hellstrom, B. Paul, J. Woodhead, and J. Hergt, “Iolite: Freeware for the visualisation and Processing of Mass Spectrometric Data,” Journal of Analytical Atomic Spectrometry 26 [2011]: 2508–18) using NIST 612 glass as an external reference and the Si concentrations determined by EDS as an internal reference. Samples were rastered on lines to determined average trace element concentrations of both pastes and glazes.
J. Victor Owen, “A New Classification Scheme for Eighteenth-Century American and British Soft-Paste Porcelains,” Ceramics in America, edited by Robert Hunter (Hanover, N.H.: University Press of New England for the Chipstone Foundation, 2007), pp. 121–40.
These stubby crystals are more silicious (60% versus 51–53% SiO2) and less aluminous (33% versus 42–44% Al2O3) than metakaolin. Mullite is much more aluminous than either of these minerals (71.8% Al2O3, 28.2% SiO2). The metakaolin analysis in Table 4 is from N. Torres Castellanos, J. Torres Agredo, and R. Majía de Gutiérrez, “Performance under Sulfate Attack of Concrete Additioned with Fluid Catalytic Cracking Catalyst Residue (FCC) and Metakaolin (MK),” Ingeniería e Investigación 33, no. 1 (2013): 18–22.
Incompatible elements (or components) are those that are preferentially concentrated in the melt phase during partial melting (and crystallization); the converse is true for compatible elements, which are partitioned into minerals as they crystallize from a melt (and are retained by minerals during low degrees of partial melting).
Martin Fisch, “Crystal Chemistry of Boron-Bearing Mullite-Type Compounds,” Ph.D. diss., Universität Bern, 2011.
Jorge Martín-Márquez, Jesús Ma. Rincón, and Maximina Romero, “Mullite Development on Firing in Porcelain Stoneware Bodies,” Journal of the European Ceramic Society 30, no. 7 (May 2010): 1599–1607.
Guilherme A. R. Gualda and Mark S. Ghiorso, “MELTS_Excel: A Microsoft Excel-Based MELTS Interface for Research and Reaching of Magma Properties and Evolution,” Geochemistry, Geophysics, Geosystems 16, no. 1 (January 2015): 315–24. Rocks and ceramic materials melt over a range of temperatures, starting at a eutectic (thermal minimum). The liquidus is the temperature above which the sample is entirely molten. On the liquidus, crystals start to form from the liquid as it cools, and tracks the liquidus surface down-temperature toward the eutectic (unless, by happenstance, the sample already has a minimum melt composition).
This temperature was calculated for a pressure of 1 bar (as would be the case in a kiln). We assumed relatively oxygenating conditions, so used the HM buffer. MELTS indicated an oxygen fugacity of -4.97 at the calculated liquidus temperature for the glaze.
For example, it is commonly stated that true (“Chinese type”) porcelain is fired at a temperature of about 1450°C. References include historical reviews in refereed journals, such as Keven J. Anderson, “Porcelain,” MRS Bulletin 15, no. 7 (July 1990): 71–72, and Lydia H. Liu, “Robinson Crusoe’s Earthenware Pot,” Critical Inquiry 25, no. 4 (Summer 1999), 728–57; and in reference books, e.g., Lesley Acton and Paul McAuley, Repairing Pottery & Porcelain: A Practical Guide, 2nd ed. (London: A & C Black, 2003). It also appears in Encyclopaedia Britannica online, at http://www.britannica.com/art/porcelain.
See J. Victor Owen and Jacob J. Hanley, “Bartlam Porcelain Re-created,” American Ceramic Circle Journal 19 (forthcoming).
Other than its lower sodium content, this glaze is not radically different from some igneous rocks (e.g., some anorthosites).
Lead is not included among the components modeled by current versions of MELTS.
Owen and Hunter, “Too Little, Too Late,” p. 339.
J. Victor Owen, “What Analytical Data Can (and Cannot) Tell Us about 18th Century Fine Ceramics,” English Ceramic Circle Transactions 22 (2010): 215–30.
E.g., W.R.H. Ramsay and E. G. Ramsay, “A Classification of Bow Porcelain from First Patent to Closure: c. 1743–1774,” Proceedings of the Royal Society of Victoria 119, no. 1 (September 2007): 1–68. Owing to its lead-rich glaze and lead-bearing S-A-C paste, many workers might classify this artifact as a type of “soft-paste” porcelain. This distinction is not important to us. We include this sample in the present investigation because this dated basket was made in the same city where the Philadelphia bowl was found, and so it begs comparison.
Owen, “A New Classification Scheme,” p. 132.
Chondrites are a type of stony meteorite. They have a primitive composition compared with crustal materials. Normalizing data to chondritic values indicates how compositionally evolved are the samples (generally rocks, but in this case, ceramics) being investigated.
See Owen, “Double Corona Structures,” p. 1258.
In marked contrast, the trace-element contents of ancient Chinese porcelains have been reported in the literature for more than a decade.
See Y. Iqbal, P. F. Messer, and W. E. Lee, “Microstructural Evolution in Bone China,” British Ceramic Transactions 99, no. 5 (2000): 193–99; and J. Victor Owen, Robert Hunter, Rod Jellicoe, and Martha Zierden, “Microstructures of Phosphatic Porcelain from Sintering to Vitrification: Evidence from Sherds Excavated in Charleston, South Carolina,” Geoarchaeology 26, no. 2 (March–April 2011): 292–313.
Diana Stradling, “Bonnin and Morris: Reading between the Lines,” in Ceramics in America, edited by Robert Hunter (Hanover, N.H.: University Press of New England for the Chipstone Foundation, 2013), pp. 191–92.
Rob Hunter, email communication, December 21, 2016.
See Owen, “Double Corona Structures,” p. 1262.
